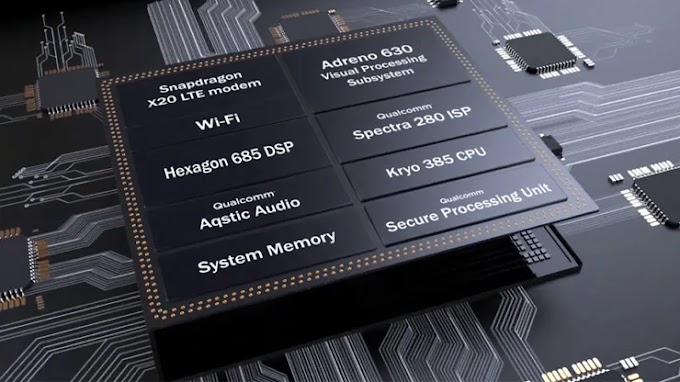
Crystal structure - Simple Cubic, Face Centered Cubic, Body Centered Cubic, Hexagonal Close Packed, Diamond, NaCl, Zincblende, CsCl, Wurzite, Perovskite
Atoms are organized in a three-dimensional regular model in a crystal in straight lines. A small part of the crystal which can be repeated in order to form the whole crystal is called a unit cell.
Devices such as transistors of solid state, lasers, solar cells, and diodes of light emitting are often produced from single crystals. Many products are polycrystaline, including most metals and ceramics. This implies that there are many small crystals crowded together where there is a random direction between the crystals. It is called an amorphous substance when the atoms of a substance are not organized in a periodic sequence. An amorphous fabric instance is glass. Although not all objects are crystals, we will waste most of our moment learning crystals as the translational symmetry makes them mathematically simpler to describe. Usually describing the conduct of more complicated components builds on the knowledge gained through the study of crystals.
Some common crystal structures you should know
Reading
Glass is an amorphous instance of fabric. Although not all things are crystals, we are going to spend most of our time studying crystals as the translational symmetry makes them easier to define mathematically. Usually describing the behavior of more complex parts builds on the understanding acquired from crystal research.
Asymmetric unit | Primitive unit cell | Conventional unit cell | Crystal |
Devices such as transistors of solid state, lasers, solar cells, and diodes of light emitting are often produced from single crystals. Many products are polycrystaline, including most metals and ceramics. This implies that there are many small crystals crowded together where there is a random direction between the crystals. It is called an amorphous substance when the atoms of a substance are not organized in a periodic sequence. An amorphous fabric instance is glass. Although not all objects are crystals, we will waste most of our moment learning crystals as the translational symmetry makes them mathematically simpler to describe. Usually describing the conduct of more complicated components builds on the knowledge gained through the study of crystals.
Some common crystal structures you should know
Simple Cubic | Face Centered Cubic | Body Centered Cubic | Hexagonal Close Packed | Diamond |
NaCl | CsCl | Zincblende | Wurzite | Perovskite |
Reading
Glass is an amorphous instance of fabric. Although not all things are crystals, we are going to spend most of our time studying crystals as the translational symmetry makes them easier to define mathematically. Usually describing the behavior of more complex parts builds on the understanding acquired from crystal research.
- For the exam you should
- know that a crystal consists of a basis (the atoms of a primitive unit cell) and one of the 14 Bravais lattices. You should be able to draw the conventional unit cell given the basis and the Bravais lattice as in this problem.
- know what the primitive lattice vectors are and how they can be used to calculate the volume of a primitive unit cell.
- The volume of a unit cell is .
- A translation vector of the crystal is where are integers.
- be able to draw the following crystal structures: simple cubic, fcc, bcc, hcp, NaCl, CsCl, hexagonal, tetragonal, and orthorhombic.
- be able to construct a Wigner Seitz cell. This is a primitive unit cell with the same symmetry as the crystal.
- know how Miller indicies are used to define directions and planes in a crystal. You should be able to draw the arrangement of atoms at the surface of a crystal cut along a plane specified by Miller indices such as in this problem.
- know what an asymmetric unit is and how it can be used to specify a crystal structure.