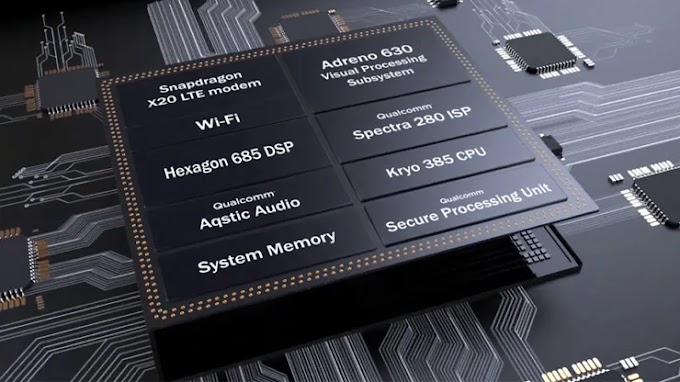
Allotropy
Allotropy, the presence of a chemical element in two or more types that may vary in the structure of atoms in crystalline solids or in the event of molecules containing distinct atom figures. The presence of various crystalline types of an object is the same event called polymorphism in the event of compounds.
Allotropes can be monotropic, in which case one of the forms is the most stable under all conditions, or enantiotropic, in which case, under different conditions, different forms are stable and undergo reversible transitions at characteristic temperatures and pressures.
Tin, coal, nitrogen, nitrogen, and oxygen are elements that exhibit allotropy. Tin and sulfur are enantiotropic: the former has a gray shape, stable below 13.2 ° C, and a white shape, stable at higher temperatures; rhombic crystals, stable below 95.5 ° C, and monoclinical crystals, stable between 95.5 ° C and melting point (119 ° C). Carbon, sulfur and nitrogen are monotropic; graphite is more fragile than rock, blue clay is more fragile than white, and diatomic oxygen with formula O2 is more viable in all normally than triatomic oxygen (ozone, O3).
Polymorphism
The capacity of a strong material to occur in more than one type or crystal structure is polymorphism in materials science. Polymorphism may be discovered in any crystalline content, including polymers, minerals, and metals, and is associated with allotropy, which relates to chemical elements. Polymorphism and other factors such as crystal habit, amorphous fraction or crystallographic defects describe the full morphology of a substance. Polymorphism is applicable to the pharmaceutical, agrochemical, pigment, colouring, food and weapons areas.
It is called packing polymorphism when polymorphism occurs as a consequence of a distinction in crystal size. Polymorphism may also lead from the presence in conformational polymorphism of distinct conformers of the same molecule. The various kinds of crystals are the consequence of hydration or solvation in pseudopolymorphism. This is called solvomorphism more properly because distinct solvents have distinct chemical formulae. Glycine, which can shape monoclinical and hexagonal crystals, is an instance of an organic polymorph. Most significant of which are: α-quartz, β-quartz, tridymite, cristobalite, moganite, coesite, and stishovite.
Minerals, calcite and aragonite, both types of calcium carbonate, are a classic illustration. Polyamorphism is an equivalent occurrence for amorphous materials when a material can undertake several distinct amorphous changes.